Advertisment
New pain target for bacterial infections
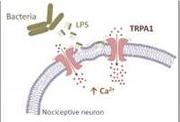
Components in the outer wall of bacteria directly activate pain sensors, triggering immediate pain and inflammatory responses. This finding by a multinational team sheds new light on pain associated with bacterial infections and reveals a new target for drugs designed to treat them.
(Bacterial components (LPS) activate the cation channel TRPA1 in sensory neurons, causing acute pain and inflammation(pictured)).
Bacterial infections are a global health problem and their treatment remains a major challenge to modern medicine. Infections of Gram-negative bacteria, in particular, are a major cause of human diseases, such as pneumonia, meningitis, gastroenteritis and gonorrhoea.
Part of Gram-negative bacteria’s danger lies in certain disease-causing components in the bacteria’s outer wall. The most significant, say the researchers, is lipopolysaccharide (LPS). In bacterial infections, LPS fragments from damaged bits of the bacterial walls are released locally, triggering an immune response.
When they come in contact with specialised TLR4 receptors at the surface of ‘sentinel’ immune cells, chemicals are released that recruit other immune cells, inducing swelling, tissue injury and eventual lyses and clearance of the bacteria.
But our immune system is unable to respond quickly enough to the presence of LPS, and fast reactions to this molecule, such as acute pain, inflammation and low blood pressure, remained unexplained until now.
The study, published in the 20 January issue of Nature Communications, uncovers, on the molecular level, how LPS causes these symptoms. The researchers found that LPS insert in the membrane surrounding sensory nerve endings, inducing a mechanical deformation that is then sensed by TRPA1 proteins. This leads to activation of TRPA1 within a matter of seconds, the influx of positively-charged ions into the nerves and the firing of electric signals that are interpreted as pain by our central nervous system.
In addition, the influx of calcium ions through TRPA1 induces the release of factors that produce dilation of the blood vessels and tissue inflammation.
The study is the culmination of a five-year probe by lead author Victor M. Meseguer (UC Berkeley) that started in a dentist’s office. Inquiring into the cause of his toothache, he was told it was a bacterial infection but that the underlying molecular mechanisms were not yet known. Today, he and his co-authors are able to show that his tooth pain was caused by bacterial LPS targeting TRPA1.
TRPA1 proteins are already known to be a detector of multiple harmful compounds contained in smoke, mustard, wasabi and tobacco. We can now add bacterial LPS to that list, say the researchers.
“The identification of TRPA1 as a molecular determinant of direct LPS effects on pain-sensing neurons offers new insights into the pathogenesis of pain and neurovascular responses during bacterial infections and opens novel avenues for their treatment,” said senior author Karel Talavera.
Contact: Karel Talavera
karel.talavera@med.kuleuven.be
32-163-30469
KU Leuven
Publication
The paper “TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins” by Victor Meseguer et al. is available on the website of Nature Communications: http://www.nature.com/ncomms/2014/140120/ncomms4125/full/ncomms4125.html
For further information go to http://www.eurekalert.org/multimedia/pub/68484.php?from=260013





